Carbon and Its Compounds Introduction PDF – Graphite, Diamond | Carbon Allotropes

Carbon
It is a member of group 14 in the Periodic Table, with symbol C and atomic number 6. It has three crystalline allotropes. It is non-metal having atomic number 6 and mass number 12. It is placed in group (IV) A or group 14 in periodic table.
Allotropy
The substance which have same chemical properties, but different physical properties are called allotrpes and this property is called allotropy. Example – Allotropies of Carbon – Diamond, Graphite, Charcoal.
1. Graphite
Also called as Plumbago or black lead. It is opaque and black. It is a very good conductor of heat and electricity. It is soft enough to form a streak on paper. It is soft, greasy, dark greyish colored crystalline solid. Its specific gravity is 2.3. The hybridization of carbon in graphite is sp2 and it has hexagonal layer structure. It is chemically more reactive than diamond. Its layer structure is held by weak van der waal’s force.
Graphite is used in making for lining and making electrodes of electric furnances, in making refractory crucibles, in making lead pencils, as a moderator in nuclear reactor, as lubricant in machinery, as a reducing agent in steel manufacturing.
Forms of Amorphous carbon obtained by destructive distillations :
| Types of Charcoal | Obtained From |
| Wood | Wood |
| Sugar | cane sugar |
| Bone or animal | animal bones |
| coke | coal |
2. Diamond
It is highly transparent and purest form of carbon. It is the hardest materials known substance. It has a very low electrical conductivity. Under normal conditions, it has the highest thermal conductivity of all known materials.Its gravity is 3.52 and refractive index 2.415.
It is chemically inert and on heating above 1500° C, transfer into graphite. It form tetrahedral crystals and hybridisation of Carbon-atom is sp3. It has high melting point (mp) and density. Black diamond called carbonado contains traces of graphite.
3. Fullerenes
It (C60) looks like a soccer ball (or bucky ball). It contains 20 six membered and 12 five membered rings of carbon atoms. It acts as wonderful lubricant and the alkali metal compounds of C60 are used as superconducting substance at the temperature range of 10-49 K.
Difference between Diamond and Graphite
| Diamond | Graphite |
| Diamond is the ultimate abrasive. | Graphite is a very good lubricant, displaying super lubricity. |
| Diamond is an excellent electrical insulator. | Graphite is a conductor of electricity. |
| It is the best known naturally occurring thermal conductor. | Some forms of graphite are used for thermal insulation (i.e., firebreak and heat shields) |
| It is highly transparent. | It is opaque. |
Graphene
Graphene is an allotrope of carbon. Its structure is one-atom-thick planar sheets of carbon atoms that are densely packed in a honeycomb crystal lattice. The term graphene was coined as a combination of graphite and the suffixene by Hanns-Peter Boehm, who described single-layer carbon foils in 1962.
Carbon Monoxide (CO)
It is formed by incomplete combustion. It is a colourless, odourless gas. It contain a triple bond and are fairly polar, resulting in a tendency to bind permanently to haemoglobin molecules. displacing oxygen, which has a lower binding affinity.
Organic Compounds
These are the compounds of mainly carbon and hydrogen or compounds of carbon and hydrogen with other elements like phosphorus, oxygen, nitrogen, sulphur, halogens etc.
Urea : is the first synthesised organic copound (by wholer).
Acetic acid : was the first organic compound synthesised in the laboratory from its elements.
Hydrocarbons and Its Type
Compounds made of carbon and hydrogen atoms only are called hydrocarbons. The natural source of hydrocarbons is petroleum.
1. Saturated hydrocarbon
The hydrocarbon in which carbon atoms are singly bonded are called saturated hydrocarbons. Saturated hydrocarbons are also called alkanes or paraffins. Alkanes are relatively unreactive under ordinary laboratory conditions. So, alkanes are also called paraffins because paraffins means little reactive. General formula of alkane – Cn H2n+2 where, n=1,2,3…
Methane is the first member of this group.
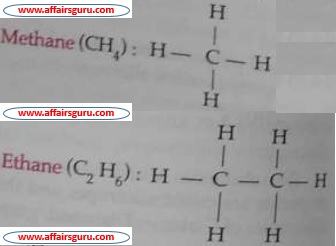
2. Unsaturated hydrocarbon
The hydrocarbon in which atoms are either doubly or triply bonded are called unsaturated hydrocarbons. Double bonded carbon atoms (C=C) hydrocarbons are called alkenes. The general formula of alkene is CnH2n
Triply bonded carbon atoms (C≡C) containing hydrocarbons are called alkynes. The general formula of alkynes are Cn H2n-2.
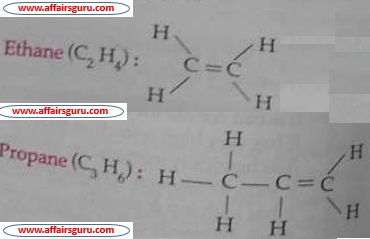
Ethylene (C2H4) is the first member of alkene and acetylene (C2H2) is the first member of alkynes respectively.
3. Aromatic hydrocarbon
These are homocyclic compounds which contains atleast one benzene ring in which carbon atoms are linked to one another by alternate single and double bonds. (4n+1) Πe- (Huckle’s rule)
In Greek, aroma stands for sweet smell. Compounds in these classification have pleasant smell. Hence they are called aromatic compounds.
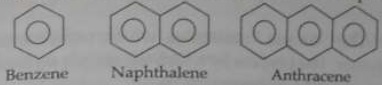
Benzene is the first member of aromatic hydrocarbons.
Functional Group
It is an atom or group of atoms in a molecule, which is responsible for the chemical properties of the molecules.
-OH is alcoholic group, -CHO is aldehyde group, =C=O is keto group, -COOH is carboxylic acid group, -O- either group.
Homologous Series
It is a series of compounds in which adjacent members differ by a -CH2 unit (14 unit mass). All members of a homologous series have same functional group and same chemical properties.
Isomerism
Compounds having the same molecular formula but different structure are called isomers and the phenomenon is called isomerism.e.g. C2H6O can have the following structure CH3OCH3 and C2H5OH.
Petroleum
The term petroleum (Latin petra = rock, oleum = oil) is the dark-coloured oily liquid with offensive odour found at various depths in many regions below the surface of the earth. It is also called rock oil, mineral oil or crude oil.
A complete list of petroleum products, approximate composition, boiling range and their uses is given ahead.
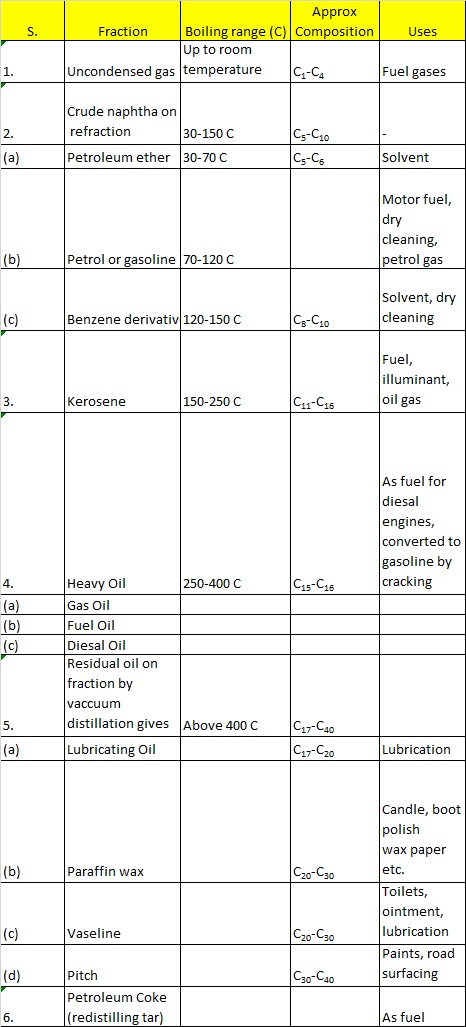
Uses of Some Important Organic Compounds
Check the below link for learning the Organic Compounds uses – Organic Compounds Uses
Methane (CH4) is used to manufacture printer ink, methyl alcohol and to obtain light and energy.
Ethylene (C2H4) is used to prepare mustard gas (war gas) and for ripening of fruits.
Glycol (C2H6O2) is used as a antifreeze mixture in car radiator and to prevent the freezing of fuel in space crafts.
Acetylene (C2H2) is used to generate light, to weld metals as oxy-acetylene flame and to prepare synthetic rubber (neoprene).
Methyl alcohol (CH3OH) is used as a fuel with petrol, used to synthesise varnish and polish, used to donature ethanol.
Chloroform (CHCl3) is used as an aneasthetic and to preserve substances obtained from plants and animals. It converts into poisonous phosgene (COCl2), when exposed to sunlight. So it is kep in dark bottles.
Glycerene (C3H8O3) is used to synthesis explosive nitroglycerine, stamp ink and boot polish.
Formic acid (HCOOH) is used as a preservative for fruits and juices, in leather industry and in coagulation of rubber.
Acetic acid (CH3COOH) is used in vinegar, medicines, and as a solvent.
Oxalic acid (C2H2O4) is used in printing of clothes, in photography and in synthesis of coaltar.
Glucose (C6H12O6) is used for the synthesis of alcohol and as a preservative for fruit juice.
Benzene (C6H6) is used as a solvent for oil fat and in dry cleaning. Sodium benzoate is a food preservative.
Toluene (C6H5CH3) is used to synthesis explosive TNT, for dry cleaning and for the synthesis of medicine like chloramine.
Phenol (C6H5OH) is used to synthesis explosive, 2, 4, 6-trinitro-phenol (picric acid) and bakelite.
Ethyl alcohol (C2H5OH) is use for drinking, in medicine to prepare tincture and as insecticide. And as a fuel with petrol.
| Name | Alochol % (Raw material) |
| Rum | 45-55% Molasses |
| Brandy | 40-50% Grapes |
| Whisky | 40-50% Barley |
| Beer | 3-6% Barley |
| Shampen | 10-15% Grapes |
| Cider | 2-6% Apple |
