Electrolysis and Law of Electrolysis, Electrochemical Cell, Battery Types
Electrolysis
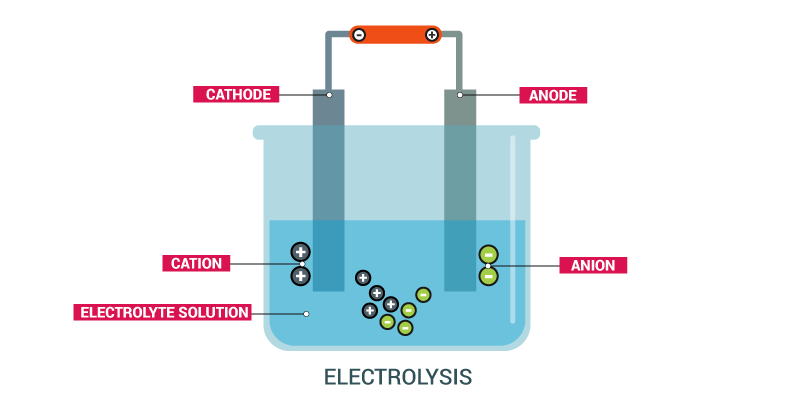
The process of chemical decomposition of an electrolyte by passage of electric current through its molten state or its solution is called electrolysis.
Electrolytes
These are the substances which allow the electricity to pass through them in their molten states or in the form of their aqueous solution and undergo chemical decomposition. Examples – acids, bases & salts. It is used
- (i) in production of oxygen for space craft and nuclear submarines.
- (ii) in layering metals to fortify them.
- (iii) in production of hydrogen for fuel.
- (iv) in electrolytic etching of metal surfaces like tools or knives with a permanent mark or logo.
Strong electrolytes
The electrolytes which are almost completely dissociated into ions in solutions are called strong electrolytes. Example – NaCl, KCl, HCL, NaOH etc.
Weak electrolytes
The electrolytes which do not ionise completely in solution and are called weak electrolytes. Example – CH3COOH, H2CO3, HCN, ZnCl2, NH4OH etc.
Electrodes
In order to pass the current through an electrolytes in molten state or in aqueous solution, two rods or plates are needed to connect with the terminal of battery. These rods or plates are called electrodes. Acid and Base Concept
Anode
The electrode which is attached to positive terminal of battery is called anode. Oxidation occurs at anode.
Cathode
The electrode which is attached to negative terminal of batteries is called cathode. Reduction occurs at cathode.
Example – Electrolysis of molten NaCl
At anode : Cl- – e → Cl
Cl + Cl → Cl2
At cathode: Na+ + e → NA
So, Cl2 gas occurs at anode while Na at cathode.
- Electrometallurgy is the process of reduction of metallic compound into pure metal by electrolysis.
- Anodisation is an electrolytic process that makes the surface of metals resistant to corrosion.
- Electrolysis of brine (the water, saturated or nearly saturated with salt, usually sodium chloride) gives hydrogen and chlorine. The products are gaseous 2NaCl + 2H2O → 2NaOH + H2 + Cl2
Fraday’s Laws of Electrolysis
There are two laws which are mentioned below.
First law of electrolysis
- It states that the quantity of elements separated by passing an electric current through a molten or dissolved salt is proportional to the quantity of electric charge passed through circuit.
w ∝ Q
w = ZQ = Z it (Because Charge = Current × time)
Second law of electrolytes
- It states that the mass of the resulting separated elements is directly proportional to the atomic masses of the elements when an appropriate integral divisor is applied.
w ∝ E or w1/w2 = E1/E2
Electrochemical Cell
- It is a device that produces an electric current from energy released by a spontaneous redox reaction (in short which converts chemical energy into electrical energy). This kind of cell includes the galvanic cell of voltaic cell.
- It has two conductive electrodes, i.e. anode (at which oxidation occurs) and cathode (at which reduction occurs).
- It contains electrolyte in between the electrodes, which contains ions that can move freely.
Battery
- It is an arrangement of one or more cells connected in series.
- It is basically a galvanic cell.
- These are of two types (i) Primary batteries (non-rechargeable) e.g, dry cell, mercury cell etc. (ii) Secondary batteries (rechargeable) e.g., lead storage battery, nickel-cadmium battery.
Lechlanche Cell or Dry Cell
- It consists of a zinc container that acts as anode and carbon (graphite) rod surrounded by powdered manganese dioxide and carbon which acts cathode.
- It contains a paste of NH4Cl and ZnCl2 in between the electrodes.
- It is used in transistors and clocks.
- It has a potential of really 1.5 V. Chemical Bonding and Chemical Reaction
Mercury Cell
- It is suitable for the low current devices like hearing aids and camera etc.
- It consists of zinc-mercury amalgam as anode and a paste of HgO and carbon as cathode. The electrolyte is a paste of KOH and ZnO.
- It has potential 1.35 V. This potential remains constant during its whole life.
Lead Storage Battery
- It is a secondary battery.
- It acts as electrochemical cell during discharging (i.e., during use) and as electrolytic cell during charging.
- It is used in automobiles and invertors.
- It consists of lead anode and a grid of lead packed with lead dioxide (PbO2) as cathode. A 38% solution of sulphuric acid is used as an electrode.
- It consists of a series of six identical cells assembled in series. Each cell may produce a potential of 2 V, hence overall voltage produced is 12 V.
PbSO4 is formed when lead storage battery is in use and lead dioxide ae formed when it is charged.
Fuel Cells
- These are galvanic cells which use energy of combustion of fuels like hydrogen (H2), methane (CH4), methanol (CH3OH) etc., as the source to produce electrical energy. e.g., hydrogen-oxygen fuel cell.
